Partnership Opportunities
Exclusive Partnering Opportunities at the 8th Gene Therapy Development Summit
As a leading event in the gene therapy space, the Gene Therapy Development Summit provided a unique platform for service providers, vendors, and solution providers to connect with key decision-makers in biotech, pharma, and academia.
Whether your focus is on manufacturing, clinical operations, or regulatory services, this was your opportunity to make targeted connections that could have transformed your business.
Why Partner with Us?
 High-Caliber Audience: Our summit brought together top decision-makers from the gene therapy community. You had the opportunity to engage with senior leaders from big pharma, biotech, and regulatory authorities, all who sought partnerships that can accelerate their gene therapy pipelines
High-Caliber Audience: Our summit brought together top decision-makers from the gene therapy community. You had the opportunity to engage with senior leaders from big pharma, biotech, and regulatory authorities, all who sought partnerships that can accelerate their gene therapy pipelines
 Focused Networking: Unlike larger conferences, this summit prioritized quality over quantity, which offered you direct access to the stakeholders that matter most. We provided opportunities to build long-term relationships that could have positioned your company as a leader in this evolving landscape
Focused Networking: Unlike larger conferences, this summit prioritized quality over quantity, which offered you direct access to the stakeholders that matter most. We provided opportunities to build long-term relationships that could have positioned your company as a leader in this evolving landscape
 Showcase Your Expertise: Whether you specialize in scaling manufacturing, regulatory guidance, or clinical development, this summit allowed you to demonstrate how your services could address critical bottlenecks in gene therapy development. Companies sought CRO services, bioanalytics, and CMC capabilities to advance their therapies through trials and into the commercial market
Showcase Your Expertise: Whether you specialize in scaling manufacturing, regulatory guidance, or clinical development, this summit allowed you to demonstrate how your services could address critical bottlenecks in gene therapy development. Companies sought CRO services, bioanalytics, and CMC capabilities to advance their therapies through trials and into the commercial market
Who Was in the Room?
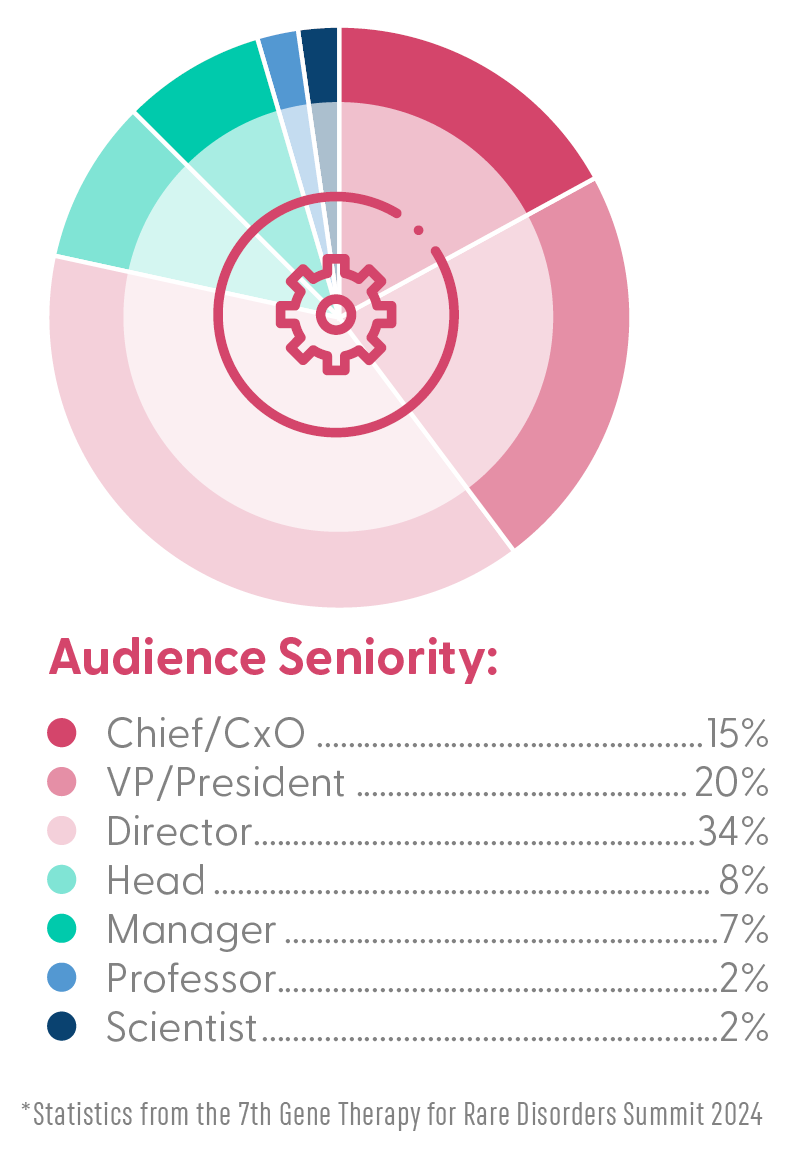
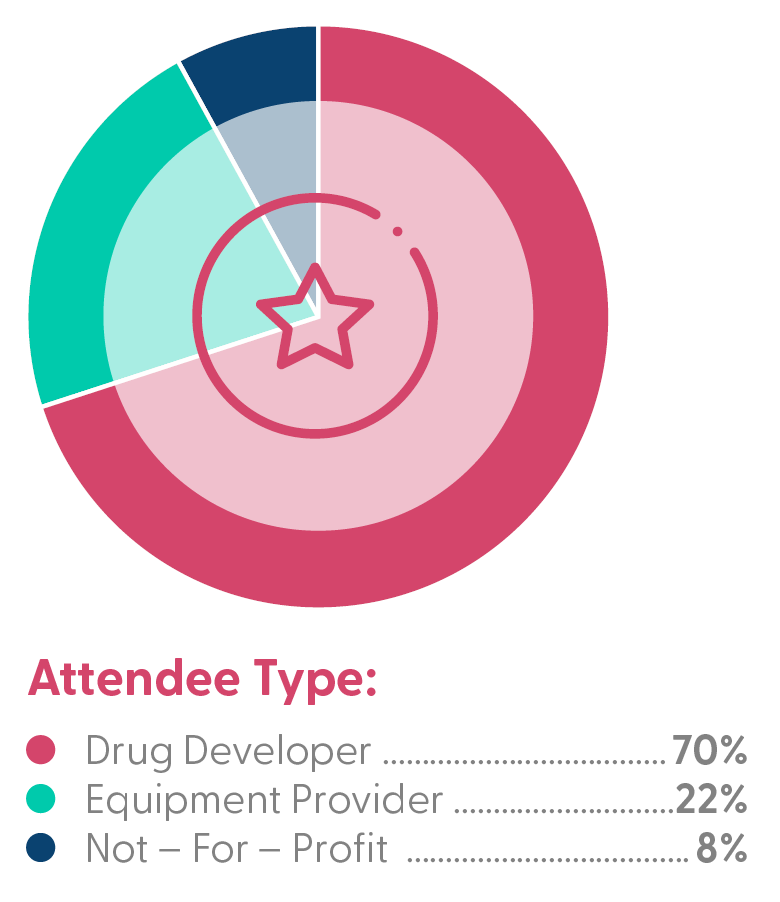
 Partner with Leading Biopharma: With over 345 gene therapy trials expected to conclude within the next five years, the demand for innovative solutions is skyrocketing. This summit positions you at the forefront of these discussions, providing visibility into the latest developments in gene therapy research, clinical trials, and commercialization
Partner with Leading Biopharma: With over 345 gene therapy trials expected to conclude within the next five years, the demand for innovative solutions is skyrocketing. This summit positions you at the forefront of these discussions, providing visibility into the latest developments in gene therapy research, clinical trials, and commercialization
By partnering with us, you’ll engage with the senior biopharma audience actively seeking solutions to advance their gene therapy programs.
Explore Other Related Events
The Gene Therapy Event Series spans in-depth content for each role and specification in gene therapy drug development, so you can curate your event calendar to meet industry leaders most relevant to your area of expertise across the year. Download our Partnership Prospectus now to find out more.





